Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare lymphoma that seems to develop mostly in women with Allergan Biocell textured implants and textured tissue expanders. Currently, there are 573 unique cases of BIA-ALCL worldwide and of those, 481 have been directly linked to Allergan textured implants.
The U.S. Food and Drug Administration (FDA) regards the risk for BIA-ALCL to be low since textured implants only account for 10 percent of all breast implants sold in the nation and globally, up to 11 million women have undergone breast augmentation and approximately 1.5 million will do so every year with little to no complication.
When diagnosed early, BIA-ALCL can be successfully treated by surgically removing the breast implant and its capsule. In later stages, if the cancer has spread, treatment includes implant and capsule removal, along with more aggressive methods like chemotherapy.
What is breast implant-associated anaplastic large cell lymphoma (BIA-ALCL)?
BIA-ALCL is a T-cell lymphoma in non-Hodgkin’s form. It is a cancer of the cells of the immune system and though the cancer cells are often found near the breast implant, within the fibrous scar capsule that surrounds it, BIA-ALCL is not a breast cancer because the cells don’t affect breast tissue.
This illustration from the FDA shows the location of BIA-ALCL within a breast.
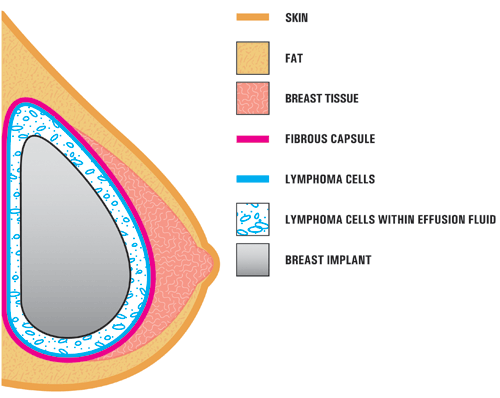
In most cases, ALCL cells are found in the fluid surrounding the implant (seroma) or are contained within the scar capsule. If untreated, the cancer cells may spread throughout the body, usually to lymph nodes.
BIA-ALCL symptoms
BIA-ALCL progresses slowly. The time for symptoms to occur is between 3 and 14 years after breast augmentation, with the average being 8 years. The most common signs are fluid buildup around the breast implant, which can lead to pain, persistent swelling, and lumps in the breast or armpit.
Other indicators may include:
- Breast enlargement
- Redness around the breast
- Changes in the shape of the breast or breasts
- Breast asymmetry
- Skin rash
- Fatigue
- Weight loss
- Hardening of the breast (capsular contracture)
What causes breast implant-associated anaplastic large cell lymphoma?
A link between ALCL and breast implants was first discovered in 2011 and since then, the FDA has been investigating the growing number of cases.
“At this time, most data suggest that BIA-ALCL occurs more frequently following implantation of breast implants with textured surfaces rather than those with smooth surfaces,” said the FDA, adding that implant fill (saline vs. silicone) seems to play no role or increase one’s risk.
It’s not well understood why BIA-ALCL forms near textured breast implants but experts believe their greater surface area and porous shell could make them ripe for bacteria formation and growth. If the bacteria trigger an infection, the body can develop an immune response (chronic inflammation) that eventually leads to BIA-ALCL.
Other potential risk factors include reactions to implant material, genetics, and long-term allergies.
What’s my risk of developing BIA-ALCL?
In the United States, the FDA estimates the lifetime risk of BIA-ALCL to be somewhere at 1 in 3,817 to 1 in 30,000. However, in Australia, where textured implants have been more widely implanted, the Australian Government Department of Health Therapeutic Goods Administration places the risk of BIA-ALCL higher, at between 1 in 1,000 and 1 in 10,000.
Should I be concerned if I have textured implants?
Textured implants with larger surface areas like Allergan Biocell texture, Silimed Polyurethane, and Mentor Siltex texture may increase a woman’s risk for BIA-ALCL, but Allergan seems to carry a risk 6 times greater than any other manufacturer.
“Although the overall incidence of BIA-ALCL appears to be relatively low, once the evidence indicated that a specific manufacturer’s product appeared to be directly linked to significant patient harm, including death, the FDA took action to alert the firm to new evidence indicating a recall is warranted to protect women’s health,” said Dr. Amy Abernethy, the FDA’s principal deputy commission on food and drugs, in a news release July 24, 2019.
Allergan voluntarily complied with the recall and has pulled their textured implants from the market. Plastic surgeons have also been notified to halt their use of the devices.
If you have Allergan Biocell breast implants, the FDA recommends:
- If you have no symptoms, removal is not necessary due to the low risk of developing BIA-ALCL
- Be aware of the symptoms of BIA-ALCL and closely monitor your breasts for changes
- If you experience symptoms, see your health care provider for evaluation and testing
- If diagnosed, undergo implant removal and have the scar capsule removed too
- Keep up with your breast implant ID card so in the event complications occur, you have a record of who the manufacturer is and the implant model name.
If you are considering implants, the FDA has a separate set of considerations.
How is BIA-ALCL diagnosed?
The doctor evaluates medical history and collects a list of symptoms. They also perform a physical exam to check for lumps, breast thickness, fluid buildup, and infection.
A biopsy removes a sample of lymph node or skin tissue to test for lymphoma cells, and ultrasound-guided aspiration of fluid may be performed to extract fluid from the scar capsule. Imaging tests, such as ultrasound and MRI, and blood tests are also commonly used to detect fluid accumulation, lumps in the breast, and swollen lymph nodes.
How is BIA-ALCL treated?
If the cancer cells are localized and are only around the implant, explantation surgery is performed to remove the implants and scar capsule (capsulectomy). This typically leads to a vast improvement of symptoms.
If the cancer has spread, a capsulectomy is performed, infected lymph nodes are removed, and further treatment can include chemotherapy, radiation therapy, and/or stem cell transplant therapy.
References:
FDA. (2019). FDA takes action to protect patients from risk of certain textured breast implants; requests Allergan voluntarily recall certain breast implants and tissue expanders from market. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan
FDA. (2019). The FDA Takes Action to Protect Patients from Risk of Certain Textured Breast Implants; Requests Allergan Voluntarily Recall Certain Breast Implants and Tissue Expanders from the Market: FDA Safety Communication. Retrieved from https://www.fda.gov/medical-devices/safety-communications/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan
FDA. (2019). Questions and Answers about Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Retrieved from https://www.fda.gov/medical-devices/breast-implants/questions-and-answers-about-breast-implant-associated-anaplastic-large-cell-lymphoma-bia-alcl
Cleveland Clinic. (2019). Breast Implant-Associated Anaplastic Large Cell Lymphoma. Retrieved from https://my.clevelandclinic.org/health/diseases/21078-breast-implant-associated-anaplastic-large-cell-lymphoma
FDA. (2019). Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) — Letter to Health Care Providers. Retrieved from https://www.fda.gov/medical-devices/letters-health-care-providers/breast-implant-associated-anaplastic-large-cell-lymphoma-bia-alcl-letter-health-care-providers